Lost at Sea Created by Suzanne Reeve, Bill Palmer, Keith Onstot & Lisa George
Why is it bad for people to drink saltwater?
The boys were finally rescued by a fishing boat, so far out in the Pacific that medical help was 48 hours away. (CNN.com) If you were the fishing boat crew, how would you treat the boys?
We use this story to frame the challenge, which is twofold: (1) Explain, using scientific understandings, why it was bad for the boys to drink saltwater. (2) Design a treatment (oral rehydration solution) to help them recover from their ordeal.
Stated in another way – put yourself in the shoes of the fisherman who rescued the boys; if medical help is far away, how would you treat them in the meantime? And what understandings of biology and chemistry would help you decide?
Unit Launch
The unit begins with a discussion about what people should and should not drink when they’re thirsty. Students share ideas and the teacher probes for initial ideas without evaluating. The story about the three boys is introduced via slides and a video news clip. Students draw & share initial ideas & questions about why someone can’t drink saltwater to stay alive and what might be happening inside the boys’ bodies over time.
Students generate a list of ideas about what they need to know to complete the challenges:
- design an oral rehydration therapy (ORT) to treat a severely dehydrated person
- create a pocket guide to making an ORT
- be able to explain scientifically why your solution works
Midpoint Check
After each concept or activity in the unit, we check back to see how what we have learned relates to the oral rehydration solution. Students take quizzes throughout the unit to assess understanding of content: calculating solution concentration, creating solutions of specific concentrations, and various kinds of membrane transport (osmosis, diffusion, active/passive transport).
Culminating Experience
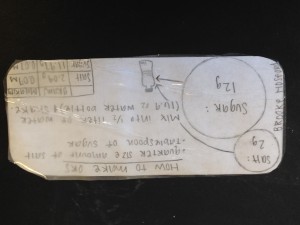
Students use the information from the lessons and a final reading to design their oral rehydration solution. Each group creates an index card containing both step-by-step visual and written directions for making the solution, as well as an explanation of what evidence the group members have that their solution will effectively treat dehydration.
Differentiation (e.g. Special Education, English Language Learners)
Differentiating the final product:
Assign each student/group a different sized container to make their solution – for example, a 1 L Nalgene bottle, a 12 oz soda can, a 20 oz empty plastic water bottle, etc. Students must take the container’s volume into account as they calculate the concentration of their solutions. More advanced students/groups can be assigned containers that require unit conversions and whose size are not round numbers (12 oz, 357 mL). A 1 L container makes the calculations more straightforward.
Additional supports for students with disabilities or students learning English:
Conversion Problems-with-molarity-color-coded-v3solutions membranes vocab list
In addition to creating their oral rehydration solution guide, students also take traditional tests and quizzes during the unit (on calculating molarity, creating solutions, and membrane transport).
Oral Rehydration Therapy Reading
Oral Rehydration Final Task and Rubric
Sample student work



Teacher Reflection
Here’s what I really enjoy about this unit:
Most students know that saltwater is bad to drink, but they typically do not understand the biology and chemistry concepts behind it. This unit showcases the connections between biology and chemistry in the human body, and there are many places in it to connect to health care professions and everyday health understandings. For example – why aren’t IV solutions made of pure water? How can you die of water poisoning? What are the “electrolytes” advertised in sports drinks?
Here’s what I’m still working on making better about this unit:
The content standards require that students have a high level of skill with molarity calculations and creating solutions. To get to this level, we have to spend a few days practicing problems, which means departing for a while from the problem context. I’m always looking for ways to keep the story context alive and continually threaded through the work.
Student Reflection
Here’s what I really enjoy about this unit:
Here’s how this unit could help me learn more effectively:
Authentic Problem
During the unit, we introduce multiple authentic contexts for treating dehydration – childhood deaths from diarrhea/dehydration in developing countries, treating yourself or others in a case of food poisoning, and rehydrating after intense physical activity. Medical professionals must also calculate solutions and understand effects of solution concentration on living cells when administering and prescribing medication.
Authentic Assessment
Students create their own solution design, along with directions and corresponding to a rubric that grades not only on content but on usability. Creating the solution requires students to integrate and apply their ideas, making this assessment also an authentic measure of their understanding and skills in context.
Student Voice
Throughout the unit, the material is connected to students’ own experiences with exercise and health, and their ideas are sought out. In the final challenge, students design their own solutions, with a minimum of teacher intervention.
Expertise
Students build their own expertise and draw on the expertise of others in their work. It would be great to bring in a parent or other community member with health care expertise to share how a knowledge of solution concentrations and cell membrane transport is important in their work. This same expert could support students as they make their final products – or perhaps an expert in global health who could give advice about how to communicate information on the index card for people in developing countries, where dehydration from diarrhea and other diseases is a significant cause of death. Students could help identify people or organizations with this expertise.
Culturally Responsive Instruction
The phenomenon of feeling thirsty and getting “dehydrated” is one that virtually all students are familiar with and have an intuitive understanding of. Connecting the content to student experience increases its relevance. A focus on impacts of dehydration and diarrhea in developing countries connects this topic to global issues, as well.
Collaboration
Students work together to solve the final challenge of designing their solution. A variety of different skills are required, including reading and comprehending scientific text, doing mathematical calculations, and “translating” a technical procedure into terms that can be easily understood and used. Students may work in groups of four or in partners; working in partners allows for greater engagement of each student.
Academic Discourse
Students are held accountable for correctly using key scientific terms, such as osmosis, diffusion, molarity, solute, hypertonic, hypotonic, and isotonic. The index card challenge also requires them to practice communicating scientific information in practical, everyday language.
NGSS HS1-PS1.A – Matter and its Interactions:The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms.
NGSS HS1-LS1.A – Structure and Function of Organisms: Systems of specialized cells within organisms help them perform the essential functions of life.
NGSS Science and Engineering Practices –
- Defining problems and solutions
- Using mathematics and computational thinking
- Engaging in argument from evidence
- Obtaining, evaluating, and communicating information
AP Biology Essential Knowledge 2.B.1: Cell membranes are selectively permeable due to their structure.
AP Biology Essential Knowledge 2.B.2: Growth and dynamic homeostasis are maintained by the constant movement of molecules across membranes.
AP Chemistry Essential Knowledge 2.A.3: Solutions are homogenous mixtures in which physical properties are dependent on the concentration of the solute and the strengths of all interactions among the particles of the solutes present.
About the Authors

Suzanne Reeve
Suzanne is a science teacher at Sammamish High School. Before teaching, she earned her Ph.D at the University of Washington in Learning Sciences, studying the everyday science understandings of young people in a diverse community.

Bill Palmer
Bill is a science teacher and instructional technology curriculum leader at Sammamish High School. He has taught for over 15 years, including two years in Europe supervising science curriculum for schools supported by the US Department of Defense.

Keith Onstot
Keith teaches science and AVID at Sammamish. He has a degree in biochemistry from the University of Washington, and advises the school’s Environmental Warriors club.

Lisa George
Lisa is a registered dietitian and science teacher who pioneered the integrated biology/chemistry courses at Sammamish and established a successful student-maintained aquaponics program. She retired from Sammamish in 2012.